| ACIDS
[��] |
|||||
| Description | ��Ī | �� |
Description | ��Ī | �� |
| Hydrochloric, N | ��ȭ����, N | 0.1 |
Formic, 0.1N | ������, 0.1N | 2.3 |
| Hydrochloric, 0.1N | ��ȭ����, 0.1N | 1.1 |
Lactic, 0.1N | ����(��Ʈ��), 0.1N | 2.4 |
| Hydrochloric, 0.01N | ��ȭ����, 0.01N | 2.0 |
Acetic, N | �ʻ�, N | 2.4 |
| Sulfuric, N | Ȳ��, N | 0.3 |
Acetic, 0.1N | �ʻ�, 0.1N | 2.9 |
| Sulfuric, 0.1N | Ȳ��, 0.1N | 1.2 |
Acetic, 0.01N | �ʻ�, 0.01N | 3.4 |
| Sulfuric, 0.01N | Ȳ��, 0.01N | 2.1 |
Benzoic, 0.01N | �Ƚ����, 0.01N | 3.1 |
| Orthophosphoric, 0.1N | �������λ�, 0.1N |
1.5 |
Alum, 0.1N | ����, 0.1N |
3.2 |
| Sulfurous, 0.1N | ��Ȳ��, 0.1N | 1.5 |
Carbonic (saturated) | ź��(��ȭ����) | 3.8 |
| Oxalic, 0.1N | �����, 0.1N | 1.6 |
Hydrogen sulfide, 0.1N | Ȳȭ����, 0.1N | 4.1 |
| Tartaric, 0.1N | Ÿ��Ÿ����, 0.1N | 2.2 |
Arsenious (saturated) | �ƺ��(��ȭ����) | 5.0 |
| Malic, 0.1N | �����, 0.1N | 2.2 |
Hydrocyanic, 0.1N | �þ�ȭ���һ�(û��), 0.1N | 5.1 |
| Citric, 0.1N | ������, 0.1N | 2.2 |
Boric, 0.1N | �ػ�, 0.1N | 5.2 |
| BASES
[����] |
|||||
| Sodium hydroxide, N | �����Ҵ�, N |
14.0 |
Ammonia, N | �ϸ�Ͼ�, N |
11.5 |
| Sodium hydroxide, 0.1N | �����Ҵ�, 0.1N | 13.0 |
Ammonia, 0.1N | �ϸ�Ͼ�, 0.1N | 11.1 |
| Sodium hydroxide, 0.01N | �����Ҵ�, 0.01N | 12.0 |
Ammonia, 0.01N | �ϸ�Ͼ�, 0.01N | 10.6 |
| Potassium hydroxide, N | ����ȭĮ��, N | 14.0 |
Potassium, 0.01N | Į��, 0.01N | 11.0 |
| Potassium hydroxide, 0.1N | ����ȭĮ��, 0.1N | 13.0 |
Magnesia (saturated) | ��ȭ���׳�(��ȭ����) | 10.5 |
| Potassium hydroxide, 0.01N | ����ȭĮ��, 0.01N | 12.0 |
Sodium sesquicarbonate, 0.1N | ź�곪Ʈ��,0.1N | 10.1 |
| Sodium metasilicate, 0.1N | �Ի�ȭ��Ʈ��, 0.1N | 12.6 |
Ferrous hydroxide (saturated) | ����ȭ����ö(��ȭ����) | 9.5 |
| Lime (saturated) | ��ȸ(��ȭ����) | 12.4 |
Calcium carbonate (saturated) | ����ȭĮ��(��ȭ����) | 9.4 |
| Sodium triphosphate, 0.1N | ���λ곪Ʈ��, 0.1N | 12.0 |
Borax, 0.1N | �ػ�, 0.1N | 9.2 |
| Sodium carbonate, 0.1N | ź�곪Ʈ��, 0.1N | 11.6 |
Sodium bicarbonate, 0.1N | ��ź�곪Ʈ��, 0.1N | 8.4 |
| BIOLOGICAL
MATERIALS [��ü ����] |
|||||
| Blood, plasma, human | ����, ����(�ΰ�) | 7.3-7.5 | Duodenal contents, human | ���������(�ΰ�) | 4.8-8.2 |
| Spinal fluid, human | ô����(�ΰ�) | 7.3-7.5 | Feces, human | �輳��(�ΰ�) | 4.6-8.4 |
| Blood, whole, dog | ����(��) | 6.9-7.2 | Urine, human | �Һ�( �ΰ�) | 4.8-8.4 |
| Saliva, human | Ÿ��(�ΰ�) | 6.5-7.5 | Milk, human | ��(�ΰ�) | 6.6-7.6 |
| Gastric contents, human | ����(�ΰ�) | 1.0-3.0 | Bile, human | ����(�ΰ�) | 6.8-7.0 |
| FOODSTUFFS
[�ķ�ǰ] |
|||||
| Apples | ��� | 2.9-3.3 | Milk, bovine | ����,�Ұ����� | 6.3-6.6 |
| Apricots | �챸 | 3.6-4.0 | Olives | �ø��� | 3.6-3.8 |
| Asparagus | �ƽ��Ķ�Ž� | 5.4-5.8 | Oranges | ������ | 3.0-4.0 |
| Bananas | �ٳ��� | 4.5-4.7 | Oysters | ��,�������� | 6.1-6.6 |
| Beans | �� | 5.0-6.0 | Peaches | ������ | 3.4-3.6 |
| Beers | ���� | 4.0-5.0 | Pears | �� | 3.6-4.0 |
| Blackberries | �������� | 4.9-5.5 | Peas | �ϵ��� | 5.8-6.4 |
| Bread, white | �� | 5.0-6.0 | Pickles, sour | ���ӹ�(�Ÿ�) | 3.0-3.4 |
| Beets | ������ | 4.9-5.5 | Pickles, dill | ���ӹ�(�̳�����) | 3.2-3.6 |
| Butter | ���� | 6.1-6.4 | Pimento | �Ǹ��䳪�� | 4.6-5.2 |
| Cabbage | ����� | 5.2-5.4 | Plums | �����ڵ� | 2.8-3.0 |
| Carrots | ��� | 4.9-5.3 | Potatoes | ���� | 5.6-6.0 |
| Cheese | ġ�� | 4.8-6.4 | Pumpkin | ȣ�� | 4.8-5.2 |
| Cherries | ü�� | 3.2-4.0 | Raspberries | �������� | 3.2-3.6 |
| Cider | ���̴� | 2.9-3.3 | Rhubarb | ��Ȳ | 3.1-3.2 |
| Corn | ������,� | 6.0-6.5 | Salmon | ���� | 6.1-6.3 |
| Crackers | ũ��Ŀ | 6.5-8.5 | Sauerkraut | �ұ����� ����� |
3.4-3.6 |
| Dates | ���߾��� | 6.5-8.5 | Shrimp | ���� ���� | 6.8-7.0 |
| Eggs, fresh | . | 7.6-8.0 | Soft drinks | û������ | 2.0-4.0 |
| Flour, wheat | . | 5.5-6.5 | Spinach | �ñ�ġ | 5.1-5.7 |
| Gooseberries | . | 2.8-3.0 | Squash | �������� | 5.0-5.4 |
| Grapefruit | . | 3.0-3.3 | Strawberries | ���� | 3.0-3.5 |
| Grapes | . | 3.5-4.5 | Sweet potatoes | ������ | 5.3-5.6 |
| Hominy (lye) | ���� �� ������ | 6.8-8.0 | Tomatoes | �丶�� | 4.0-4.4 |
| Jams, fruit | ���� �� | 3.5-4.0 | Tuna | ��ġ | 5.9-6.1 |
| Jellies, fruit | ���� ���� | 2.8-3.4 | Turnips | ���� | 5.2-5.6 |
| Lemons | ���� | 2.2-2.4 | Vinegar | ���� | 2.4-3.4 |
| Limes | ���� | 1.8-2.0 | Water, drinking | �� | 6.5-8.0 |
| Maple syrup | ��dz��� | 6.5-7.0 | Wines | ������ | 2.8-3.8 |
��ü�� �з�
�����ü�� ���� �Ǵ� ���� ��ü�� �з��Ǿ��� �� �ִ�. ���̴�
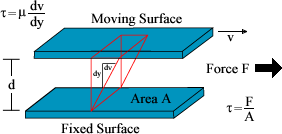
��ü�� Tangential Stress
(���� ǥ�鿡 ���� ��[����ǥ�� �̵�ǥ�鰣 ������ )�� Rate of Shear(������ �ӵ����� ���� �Ÿ��� ����)����
�����̴�.
���� �����̰� ��ü�� �ӵ���ȭ��" 0 "���� �з�(Stress)��"0"�� �̰��� ������ü�̴�.
�װ��� �ƴϸ� ���� ��ü�̰� ���� �ӵ� ��ȭ�� ���� Stress Curve�� ���� ���� ����ȭ���� �ϳ���
���� �з��Ǿ���.
���� ��ü�� ���Ͽ�, �ӵ���ȭ�� ������ �����Ѵ�; �װ��� ��ü��
�װ��� �ӵ��� ���� �����ų� ������
Stress�� ���´�. �̵� Ư���� ���� ��ü�� ����ȭ �Ǿ��� �� �ִ�.
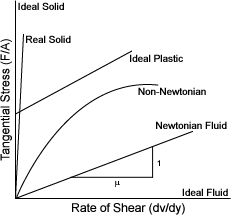
| NEWTONIAN [���� ��ü] | |
| �� ��κ��� �ұݹ� ������ ������� ������(����������) ���������� ���ָ� ���� ��κ��� ���Ϳ��� ��κ��� ���� |
 |
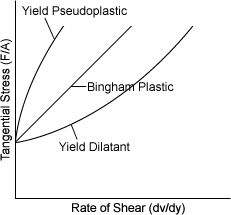
| NON-NEWTONIAN [���� ��ü] | |
| YIELD PSEUDOPLASTIC, BINGHAM PLASTIC, YIELD DILATANT |
|
| ������ (clay) ���� (Mud) Ÿ�� (Tar) ����� ������ �д� ������ �ҿ������� �����Ҽ������Ӽַ�� |
 |
| PSEUDOPLASTIC | |
| ����� ������ Paper pulp(���̺о�) Grease Soap Paint Printer's ink ����,�츻 (Starch) ����,õ��������(Latex solutions) �����,��������(Most emulsions) |
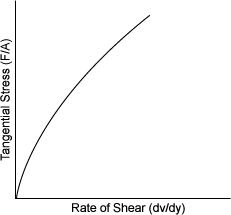 |
| DILATANT | |
| �弮(Feldspar) ���(Mica) ������(Clay) �ػ�(Beach sand) ����,ǥ��(Quicksand) ���о�(Starch in water) |
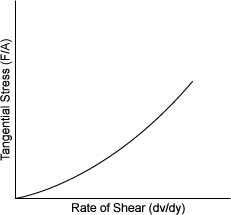 |
| THIXOTROPIC - RHEOPECTIC | |
| ��ũ ��κ��� ����Ʈ Carboxymethyl cellulose Silica gel Greases Asphalt Starch Bentonite ���������(Gypsum solutions in water) Thixotropic - decreases viscosity
over time |
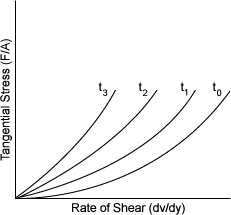 |
������ ��� ��
|
Equivalents
of Head and Pressure (in. and feet of water at 68��F, in. and feet mercury at 32��F) |
|||||||||||
| lb/in2 | lb/ft2 | Atm. | kg/cm2 | kg/m2 | in. water |
ft. water |
in. mmHg |
mm mmHg |
Bars | MPa | |
| lb/in2 | 1 | 144 | 0.068046 | 0.070307 | 703.070 | 27.7276 | 2.3106 | 2.03602 | 51.7150 | 0.06895 | 0.006895 |
| lb/ft2 | 0.006944 | 1 | 0.000473 | 0.000488 | 4088241 | 0.1926 | 0.01605 | 0.01414 | 0.35913 | 0.00048 | 0.000048 |
| Atm. | 14.696 | 2116.22 | 1 | 1.0332 | 10332.3 | 407.484 | 33.9570 | 29.921 | 760 | 1.01325 | 0.101325 |
| kg/cm2 | 14.2233 | 2048.16 | 0.96784 | 1 | 10000 | 394.38 | 32.8650 | 28.959 | 735.559 | 0.98067 | 0.098067 |
| kg/m2 | 0.001422 | 0.20477 | 0.000097 | 0.0001 | 1 | 0.03944 | 0.003287 | 0.0029 | 0.07356 | 0.00010 | 0.000010 |
| in. water |
0.036092 | 5.1972 | 0.002454 | 0.00253 | 25.375 | 1 | 0.08333 | 0.07343 | 1.8651 | 0.00249 | 0.000249 |
| ft. water |
0.432781 | 62.3205 | 0.029449 | 0.03043 | 304.275 | 12 | 1 | 0.88155 | 22.3813 | 0.02984 | 0.002984 |
| in. mmHg |
0.491154 | 70.7262 | 0.033421 | 0.03453 | 345.316 | 13.6185 | 1.1349 | 1 | 25.4001 | 0.03386 | 0.003386 |
| mm mmHg |
0.019337 | 2.78450 | 0.001316 | 0.00136 | 13.5951 | 0.53616 | 0.044680 | 0.03937 | 1 | 0.00133 | 0.000133 |
| Bars | 14.5038 | 2088.55 | 0.98692 | 1.01972 | 10197.2 | 402.156 | 33.5130 | 29.5300 | 750.062 | 1 | 0.10 |
| MPa | 145.038 | 20885.5 | 9.8692 | 10.1972 | 101972 | 4021.56 | 335.130 | 295.300 | 7500.62 | 10.0 | 1 |
���� ��
|
Flow
Equivalents for any Liquid |
||||||||
| US gpm |
Imp gpm |
US million gal/day |
sec-ft� | m3/hr | liters/sec | barrels/min | barrels/day | |
| US gpm | 1 | 0.8327 | 0.00144 | 0.00223 | 0.2271 | 0.0631 | 0.0238 | 34.286 |
| Imp gpm | 1.201 | 1 | 0.00173 | 0.002676 | 0.2727 | 0.0758 | 0.02859 | 41.176 |
| US million gal/day | 694.4 | 578.25 | 1 | 1.547 | 157.7 | 43.8 | 16.53 | 23810 |
| sec-ft� | 448.83 | 373.7 | 0.646 | 1 | 101.9 | 28.32 | 10.686 | 15388 |
| m3/hr | 4.403 | 3.67 | 0.00634 | 0.00982 | 1 | 0.2778 | 0.1048 | 151 |
| liters/sec | 15.85 | 13.20 | 0.0228 | 0.0353 | 3.60 | 1 | 0.3773 | 543.3 |
| barrels/min | 42 | 34.97 | 0.0605 | 0.0937 | 9.538 | 2.65 | 1 | 1440 |
| barrels/day | 0.0292 | 0.0243 | 0.000042 | 0.000065 | 0.00662 | 0.00184 | 0.00069 | 1 |
ü���� ���� ��
|
Volume
and Weight Equivalents Weight Equivalent Basis Water at 60�� (15.6��) |
|||||||||
| US Gallons |
Imp Gallons |
in3 | ft3 | Liters | Meters3 | Pounds | US Tons |
Kg | |
| US Gallons |
1 | 0.8327 | 231 | 0.13368 | 3.7854 | 0.0037854 | 8.338 | 0.00417 | 3.782 |
| Imp Gallons |
1.20094 | 1 | 277.39 | 0.16054 | 4.546 | 0.004546 | 10.0134 | 0.005 | 4.542 |
| in3 | 0.004329 | 0.003605 | 1 | 0.0005787 | 0.016387 | 0.000016387 | 0.036095 | 55409 | 0.016372 |
| ft3 | 7.48052 | 6.229 | 1728 | 1 | 28.317 | 0.02832 | 62.3714 | 0.03119 | 28.291 |
| Liters | 0.2642 | 0.2200 | 61.024 | 0.035315 | 1 | 0.001 | 2.2029 | 0.0011 | 0.1000 |
| Meters3 | 264.2 | 220.0 | 61024 | 35.315 | 1000 | 1 | 2202.65 | 1.10133 | 1000 |
| Pounds | 0.1199 | 0.09987 | 27.71 | 0.016033 | 0.4539 | 0.000454 | 1 | 0.0005 | 0.45359 |
| US Tons |
239.87 | 199.7 | 55409 | 32.066 | 907.9 | 0.908 | 2000 | 1 | 907.2 |
| Kg | 0.2644 | 0.2202 | 61.08 | 0.03534 | 1 | 0.001 | 2.205 | 0.0011 | 1 |
Properties
of Common Liquids |
|||
At
68�� and 14.7 psia |
|||
| Liquid | Density (lb/ft3) | Bulk Modulus | Acoustic Velocity |
| Pure Water | 62.3 | 318(s) | 4865 |
| Seawater | 63.9 | 344(s) | 4993 |
| Benzene | 54.8 | 222(s) | 4324 |
| Methanol | 49.3 | 144(s) | 3678 |
| Ethanol | 49.3 | 155(s) | 3812 |
| Turpentine | 54.2 | 223(s) | 4363 |
At
68�� and 200 psia |
|||
| Propane | 30.8 | 25 (t) | 2000-2500 |
| Isobutane | 35.0 | 41(t) | 2200-2800 |
| N-butane | 35.8 | 53(t) | 3100-3700 |
Physical Properties of Gases
(Approximate Values Calculated at 68�� and 147 psia)
cp = specific heat at constant pressure
cv = specific heat at constant volume
| Name of Gas | Chemical Formula or Symbol | Approx. Molecular
Weight M |
Weight Density,
Pounds per Cubic Foot p |
Specific Gravity
Relative to Air Sg |
Individual Gas
Constant R |
Specific Heat at Room Temperature Btu/Lb �� | Heat Capacity per Cubic Foot | k equal to cp/cv |
||
| cp | cv | cp | cv | |||||||
| Acetylene (ethyne) | C2H2 | 26.0 | .0682 | 0.907 | 59.4 | 0.350 | 0.269 | .0239 | .0184 | 1.30 |
| Air | -- | 29.0 | .0752 | 1.000 | 53.3 | 0.241 | 0.172 | .0181 | .0129 | 1.40 |
| Ammonia | H3N | 17.0 | .0448 | .0596 | 91.0 | 0.523 | 0.396 | .0234 | .0178 | 1.32 |
| Argon | Ar | 39.9 | .1037 | 1.379 | 38.7 | 0.124 | 0.074 | .0129 | .0077 | 1.67 |
| Butane | C4H10 | 58.1 | .1554 | 2.067 | 26.5 | 0.395 | 0.356 | .0614 | .0553 | 1.11 |
| Carbon dioxide | CO2 | 44.0 | .1150 | 1.529 | 35.1 | 0.205 | 0.158 | .0236 | .0181 | 1.30 |
| Carbon monoxide | CO | 28.0 | .0727 | 0.967 | 55.2 | 0.243 | 0.173 | .0177 | .0126 | 1.40 |
| Chlorine | Cl | 70.9 | .1869 | 2.486 | 21.8 | 0.115 | 0.086 | .0215 | .0162 | 1.33 |
| Ethane | C2H6 | 30.0 | .0789 | 1.049 | 51.5 | 0.386 | 0.316 | .0305 | .0250 | 1.22 |
| Ethylene | C2H4 | 28.0 | .0733 | 0.975 | 55.1 | 0.400 | 0.329 | .0293 | .0240 | 1.22 |
| Helium | He | 4.0 | .01039 | 0.1381 | 386.3 | 1.250 | 0.754 | .0130 | .0078 | 1.66 |
| Hydrogen chloride | HCl | 36.5 | .0954 | 1.268 | 42.4 | 0.191 | 0.135 | .0182 | .0129 | 1.41 |
| Hydrogen | H | 2.0 | .00523 | 0.0695 | 766.8 | 3.420 | 2.426 | .0179 | .0127 | 1.41 |
| Hydrogen sulphide | H2S | 34.1 | .0895 | 1.190 | 45.2 | 0.243 | 0.187 | .0217 | .0167 | 1.30 |
| Methane | CH4 | 16.0 | .0417 | 0.554 | 96.4 | 0.593 | 0.449 | .0247 | .0187 | 1.32 |
| Methyl chloride | CH3Cl | 50.5 | .1342 | 1.785 | 30.6 | 0.240 | 0.200 | .0322 | .0268 | 1.20 |
|
Natural gas |
-- | 19.5 | .0502 | 0.667 | 79.1 | 0.560 | 0.441 | .0281 | .0221 | 1.27 |
| Nitric oxide | NO | 30.0 | .0780 | 1.037 | 51.5 | 0.231 | 0.165 | .0180 | .0129 | 1.40 |
|
Nitrogen |
N | 28.0 | .0727 | 0.967 | 55.2 | 0.247 | 0.176 | .0180 | .0127 | 1.41 |
|
Nitrous oxide |
N2O | 44.0 | .1151 | 1.530 | 35.1 | 0.221 | 0.169 | .0254 | .0194 | 1.31 |
| Oxygen | O | 32.0 | .0831 | 1.105 | 48.3 | 0.217 | 0.155 | .0180 | .0129 | 1.40 |
| Propane | C3H8 | 44.1 | .1175 | 1.562 | 35.0 | 0.393 | 0.342 | .0462 | .0402 | 1.15 |
| Propene (propylene) | C3H6 | 42.1 | .1091 | 1.451 | 36.8 | 0.358 | 0.314 | .0391 | .0343 | 1.14 |
| Sulphur dioxide | SO2 | 64.1 | .1703 | 2.264 | 24.0 | 0.154 | 0.122 | .0262 | .0208 | 1.26 |
Note: Values for natural gas and air are representative only. Exact characteristics require knowledge of specific constituents.